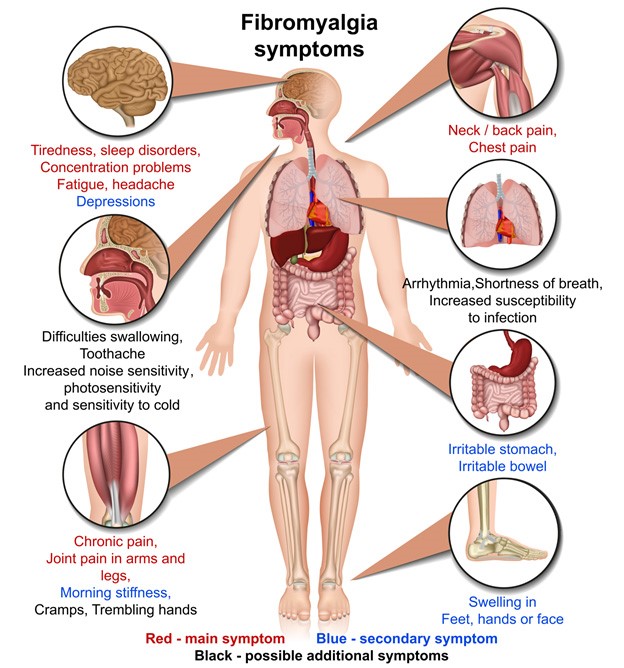
Non-opioid drug candidate from Tonix lowered pain and also improved sleep and fatigue
Latest Phase 3 trial achieved primary endpoint (p=0.00005)
Potential for FDA Approval in 2025
People who are suffering from fibromyalgia pain but don’t want to risk addiction to an opioid are closer to a new option for treatment.

Tonix Pharmaceuticals (NASDAQ:TNXP) reported positive Phase 3 study results for its non-opioid painkiller in late December. The drug lowered the characteristic widespread pain in fibromyalgia reported by study volunteers, a sign it could potentially be the first in a new class of painkillers to be approved for use in fibromyalgia.
The experimental medicine also improved sleep and fatigue in fibromyalgia, suggesting that it may reduce the use of multiple drugs – called polypharmacy – which is common in fibromyalgia.
With the current FDA-approved drugs, fibromyalgia patients face a choice: take Lyrica® and have less energy, or take Cymbala® or Savella® and have worse sleep. Tonix’s new drug, Tonmya™, improved pain, sleep and fatigue in fibromyalgia patients.
Tonix says it will file for approval from the U.S. Food and Drug Administration in the second half of this year.
“When Tonmya is approved, as we expect, it will offer a new option for patients who need pain relief but who do not want to take an opioid and can’t tolerate any of the three approved drugs,” Tonix Chief Executive Officer Seth Lederman, MD said. “For Tonmya, the most common systemic side effects – headache and somnolence – were reported in about 3% of patients. No changes to weight or blood pressure were observed, which are issues with other fibromyalgia drugs. Tonmya is a sublingual tablet, and the most common local administration side effect was tongue numbness, which was transient and self-limited. Ultimately, we believe Tonmya has the potential to be a first-line treatment for newly diagnosed patients.”
Tonix said the researchers who conducted the Phase 3 study plan to present the data at upcoming scientific meetings and hope to publish their results in a peer-reviewed journal later this year.
About 10 million Americans suffer from fibromyalgia, and the majority are prescribed medicine for the characteristic widespread pain. The three approved products to treat that symptom in fibromyalgia are Lyrica, Cymbalta and Savella. Many patients report they are hard to tolerate. As a result, many take opioids because they provide relief or because they have become addicted or dependent on them.
Reference<https://finance.yahoo.com/news/tonix-drug-shown-relieve-pain-130000206.html?guccounter=1&guce_referrer=aHR0cHM6Ly93d3cuZ29vZ2xlLmNvbS8&guce_referrer_sig=AQAAALix6sFq9veWOFi3ACp8d4bTRx7su4KG87hZONhv-5mT_NE06hYDB77MLX0o6ASb1-4S6d0_oKkGx4rd3-mFgPZ76XJirmFOM9xw1JP-cehaybclLpiLzasY_5nqTzOLvfyRifxVMIhPD9xBooNVZWYSP6yl4eJkzZY2-LeokfJ9